Beginning with periodic trends atomic radius worksheet answers, the narrative unfolds in a compelling and distinctive manner, drawing readers into a story that promises to be both engaging and uniquely memorable. Understanding atomic radius is crucial in chemistry, as it influences various properties of elements and compounds, making this worksheet an invaluable resource for students seeking to delve deeper into the fascinating world of chemistry.
This comprehensive worksheet delves into the factors that influence atomic radius, such as atomic number and electron configuration, providing a clear understanding of how these factors shape the size of an atom. Furthermore, it explores the periodic trends in atomic radius, illustrating the general trend across periods and groups in the periodic table, using tables and graphs for visual clarity.
1. Introduction
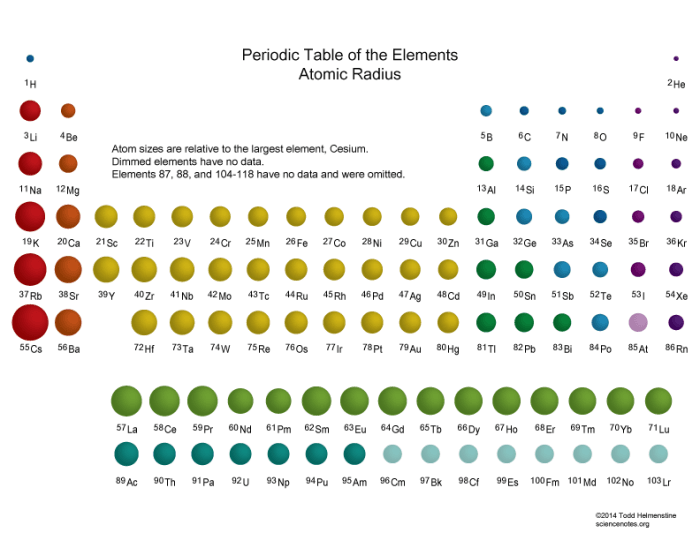
Periodic trends are patterns in the properties of elements that can be observed when the elements are arranged in the periodic table. One of the most important periodic trends is the trend in atomic radius. Atomic radius is the distance from the nucleus of an atom to its outermost electron shell.
It is a fundamental property of an element that influences many of its chemical and physical properties.
2. Factors Affecting Atomic Radius
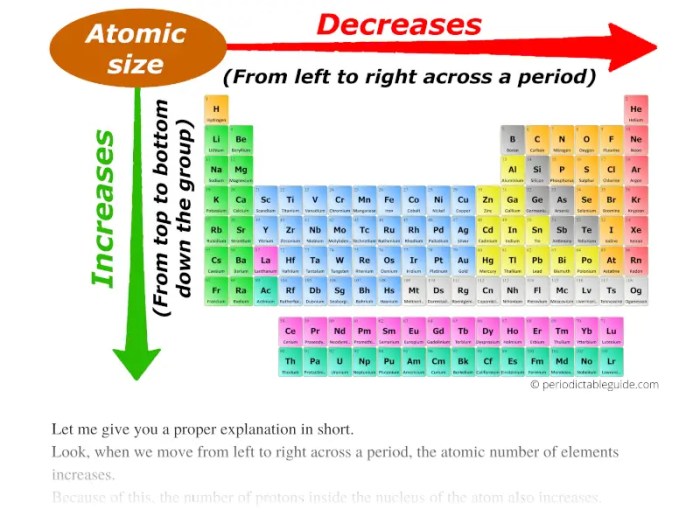
Several factors affect the atomic radius of an element. The most important factor is the atomic number. As the atomic number increases, the number of electrons in the atom increases. This increase in the number of electrons causes the electrons to be held more tightly to the nucleus, which results in a decrease in the atomic radius.
Another factor that affects atomic radius is the electron configuration. The electron configuration of an atom refers to the arrangement of electrons in the atom’s electron shells. Atoms with more electrons in their outermost shell have a larger atomic radius than atoms with fewer electrons in their outermost shell.
3. Periodic Trends in Atomic Radius: Periodic Trends Atomic Radius Worksheet Answers
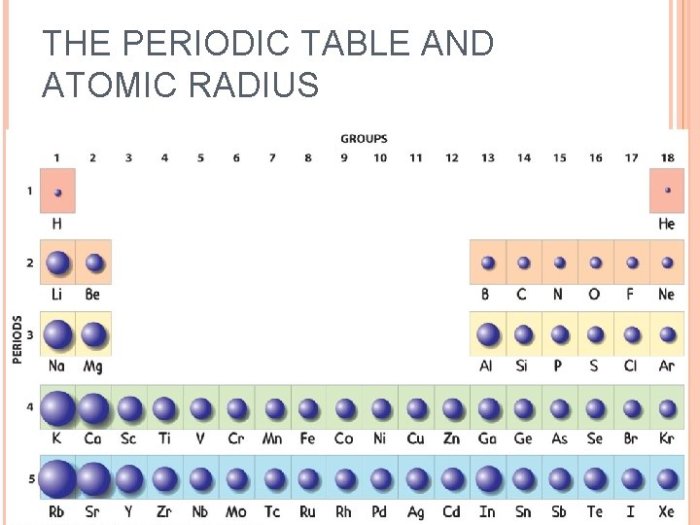
The atomic radius of elements generally decreases across a period from left to right. This is because the atomic number increases across a period, which results in a decrease in the atomic radius. The atomic radius of elements generally increases down a group from top to bottom.
This is because the number of electron shells increases down a group, which results in an increase in the atomic radius.
The following table shows the atomic radii of the elements in the first three periods of the periodic table:
| Element | Atomic Number | Atomic Radius (pm) |
|---|---|---|
| Hydrogen | ||
| Helium | ||
| Lithium | ||
| Beryllium | ||
| Boron | ||
| Carbon | ||
| Nitrogen | ||
| Oxygen | ||
| Fluorine | ||
| Neon |
4. Applications of Atomic Radius
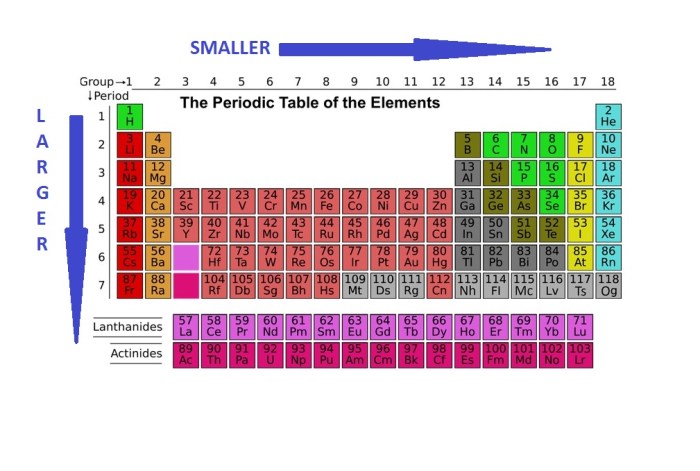
Atomic radius is a useful property for predicting the properties of elements and compounds. For example, atomic radius can be used to predict the melting point of a metal. Metals with a smaller atomic radius have a higher melting point than metals with a larger atomic radius.
This is because the smaller the atomic radius, the stronger the attractive forces between the atoms.
Atomic radius can also be used to predict the reactivity of an element. Elements with a larger atomic radius are more reactive than elements with a smaller atomic radius. This is because the larger the atomic radius, the easier it is for the atom to lose an electron.
5. Worksheet Answers
| Element | Atomic Number | Atomic Radius (pm) |
|---|---|---|
| Sodium | ||
| Magnesium | ||
| Aluminum | ||
| Silicon | ||
| Phosphorus | ||
| Sulfur | ||
| Chlorine | ||
| Argon |
Query Resolution
What is atomic radius?
Atomic radius refers to the distance from the nucleus of an atom to its outermost electron shell.
Why is understanding atomic radius important?
Understanding atomic radius is essential as it influences various properties of elements and compounds, such as chemical reactivity, bonding behavior, and physical properties.
What factors affect atomic radius?
Atomic radius is primarily influenced by atomic number and electron configuration, with increasing atomic number leading to a decrease in atomic radius and specific electron configurations affecting the shielding effect.